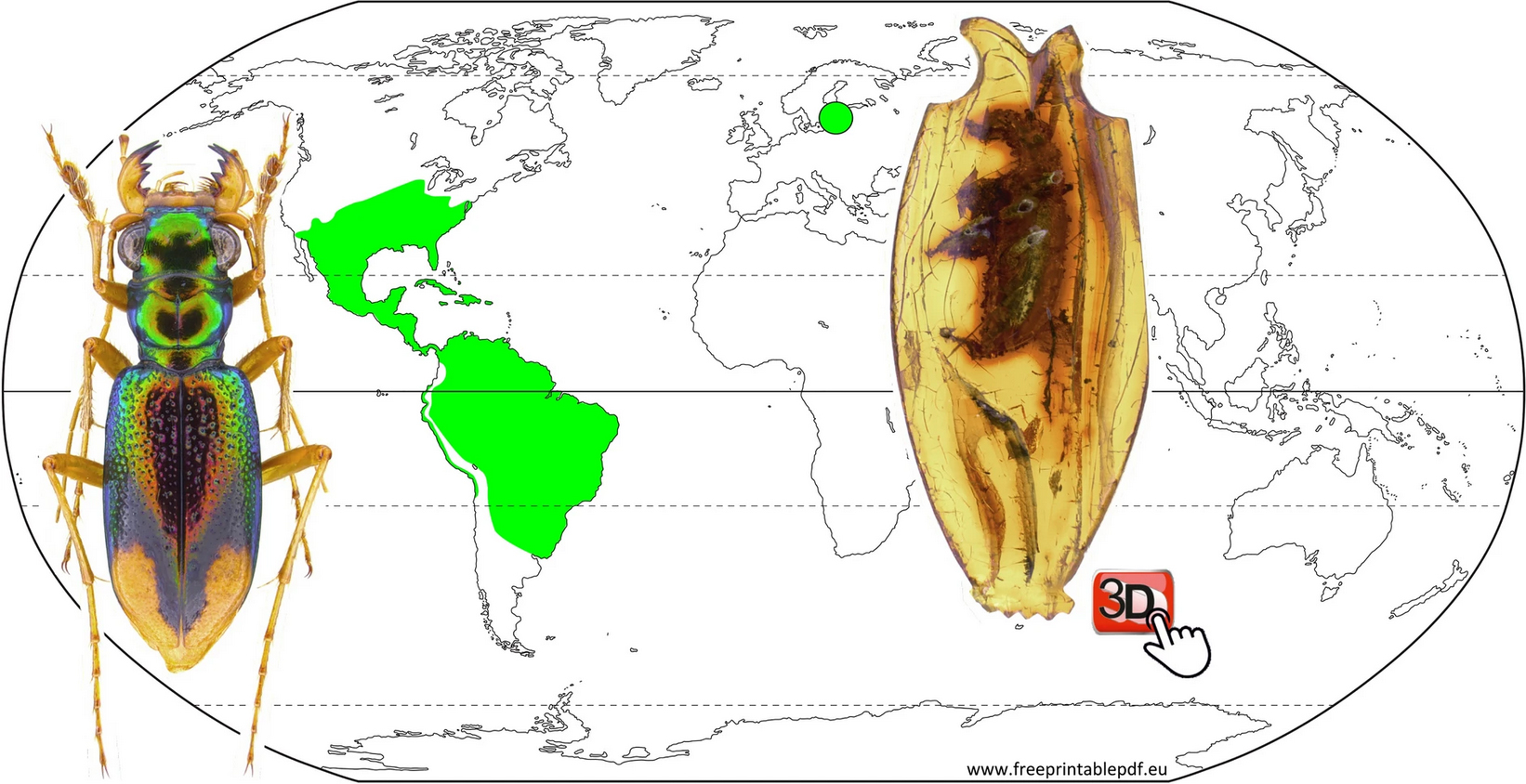
Tetracha carolina from the Essig Museum collection.
There are about 3,000 species of tiger beetles (Cicindelidae) worldwide, many of which are in decline, and several listed as endangered or threatened. Known for their speed, predatory prowess, and beauty, they are a popular group among hobbyists and an active area for research.
There are only a few known tiger beetle fossils, but one of the most cited is the extant tiger beetle Tetracha carolina (Linneus, 1763) that was identified by Walther Horn over a century ago (Deutsche Entomologische Zeitschrift, 1906: 329–336). UC Berkeley Professor Kipling Will, who recently re-studied this amber fossil, provides some insight as to why this identification prompted further questioning:
“There was this standing idea that a species of tiger beetle had persisted as a species level taxon for 30 or 40 million years without change. If you followed what Horn originally said, that this was Tetracha carolina, then you’d have to assume that there’s really been no significant evolution of this species over that long period of time which seems a little odd. We expect species turnover differences to happen at a much quicker rate than that. And the Baltic is nowhere close to the current distribution.”
Current distribution of Tetracha spp. (green shading) and location of amber fossil (green circle). From Schmidt, J., Scholz, S., Wiesner, & Will K. 2023. “MicroCT data provide evidence correcting the previous misidentification of an Eocene amber beetle (Coleoptera, Cicindelidae) as an extant species.” Scientific Reports 13, 14743. https://doi.org/10.1038/s41598-023-39158-7
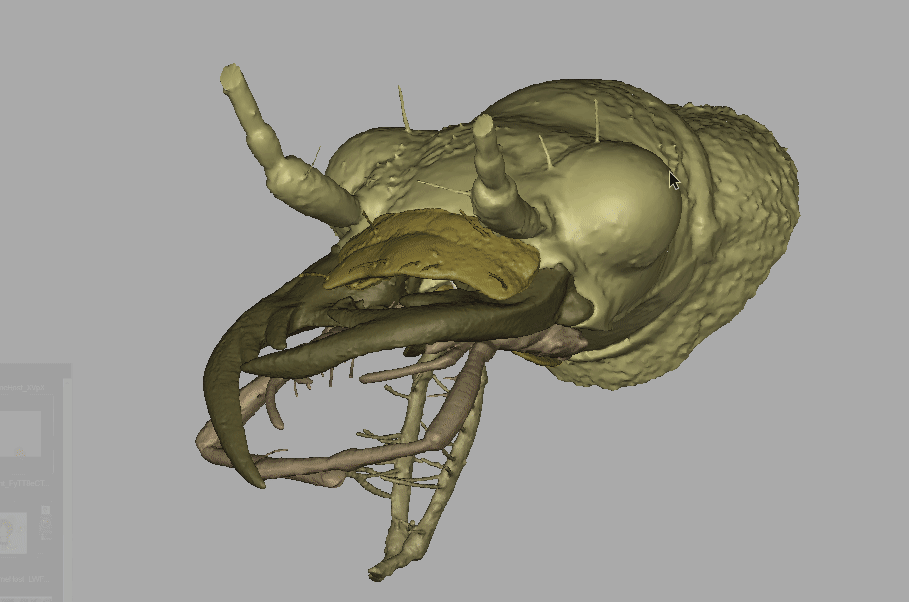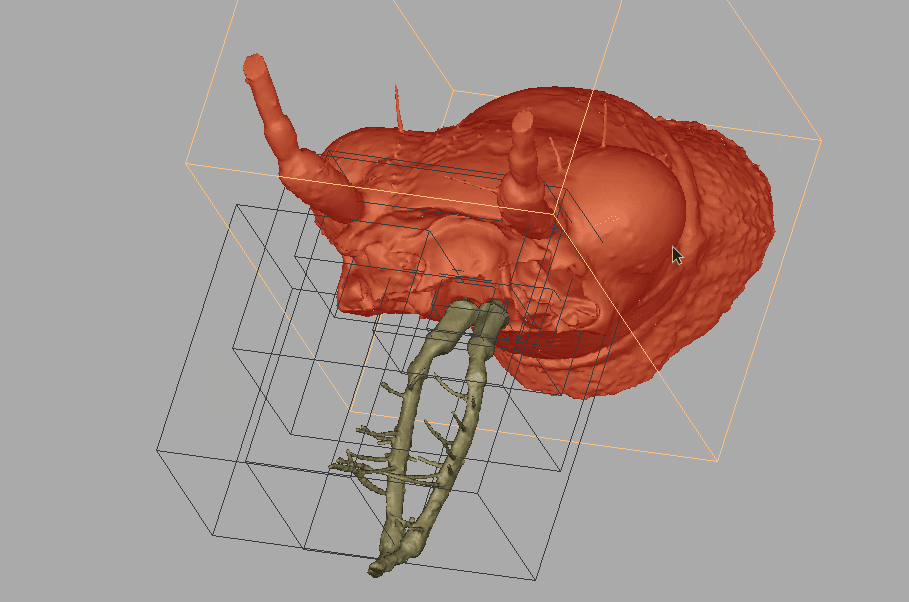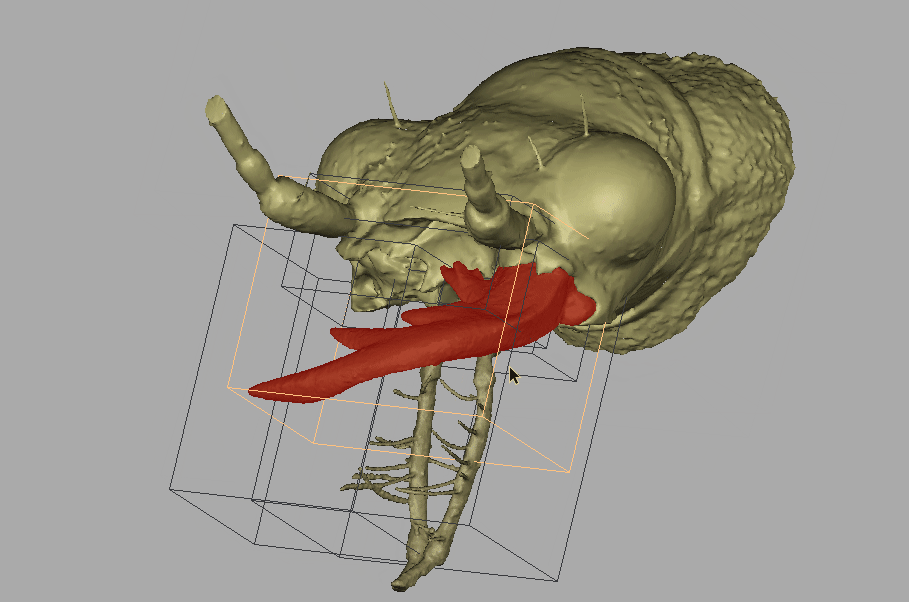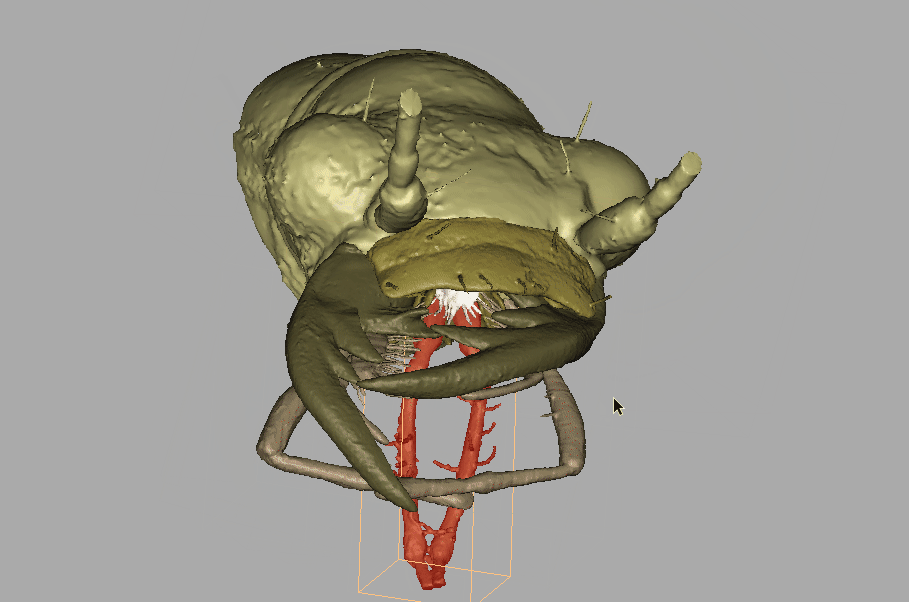
Amber fossils can be difficult to analyze due to the natural visual obstructions, such as patina flow lines, dust particles, air bubbles, and corrosion cracks. Using standard optical microscopes, researchers are also limited in what traits can be used for identification, because they can only see what is visible to the eye. However, with recent technological advances, such as MicroCT scanning and 3D imaging, detailed and interactive models of fossil specimens can be recreated. Because of this technology the team was able to challenge Horn’s identification.
Recording of the fossil specimen’s 3D model.
“With the MicroCT scanning, we can see through all of that, and then produce these images. Even though that fossil was never in my hand, I could see all of the images and I could study all the details. And once we publish it, if people in the future disagree with us, well, they have the exact same things to look at as we did.”
Will and colleagues discovered that Horn’s fossil was not an extant Tetracha, but an extinct Palaeoiresina cassolai.
“Both the age and the distribution make sense. If there are examples of things that persist unchanged for 30 or 40 million years, this is certainly not one of them. And we think that’s unlikely for pretty much any of the fossils of that age.”
Identifying fossils is important because of how other researchers will integrate the fossil data into their own work.
“We can build phylogenies and that tells us about relationships, and we can talk about relative divergence from an ancestor to all descendants. But those phylogenies are not time-calibrated. We want to look at things like diversification rates, the timing of evolution of novelties, and all the kinds of fun stuff in the context of everything else that’s happening on the planet. Like, what were the positions of tectonic plates? What was the climate like 30 million, 50 million, 100 million years ago? Correlating phylogeny and the forces that drove evolution and extinction. So having the fossils carefully studied allows us to calibrate those trees and then lets us look at that story through time. Understanding the events of the past will help us make more accurate models of the future.”
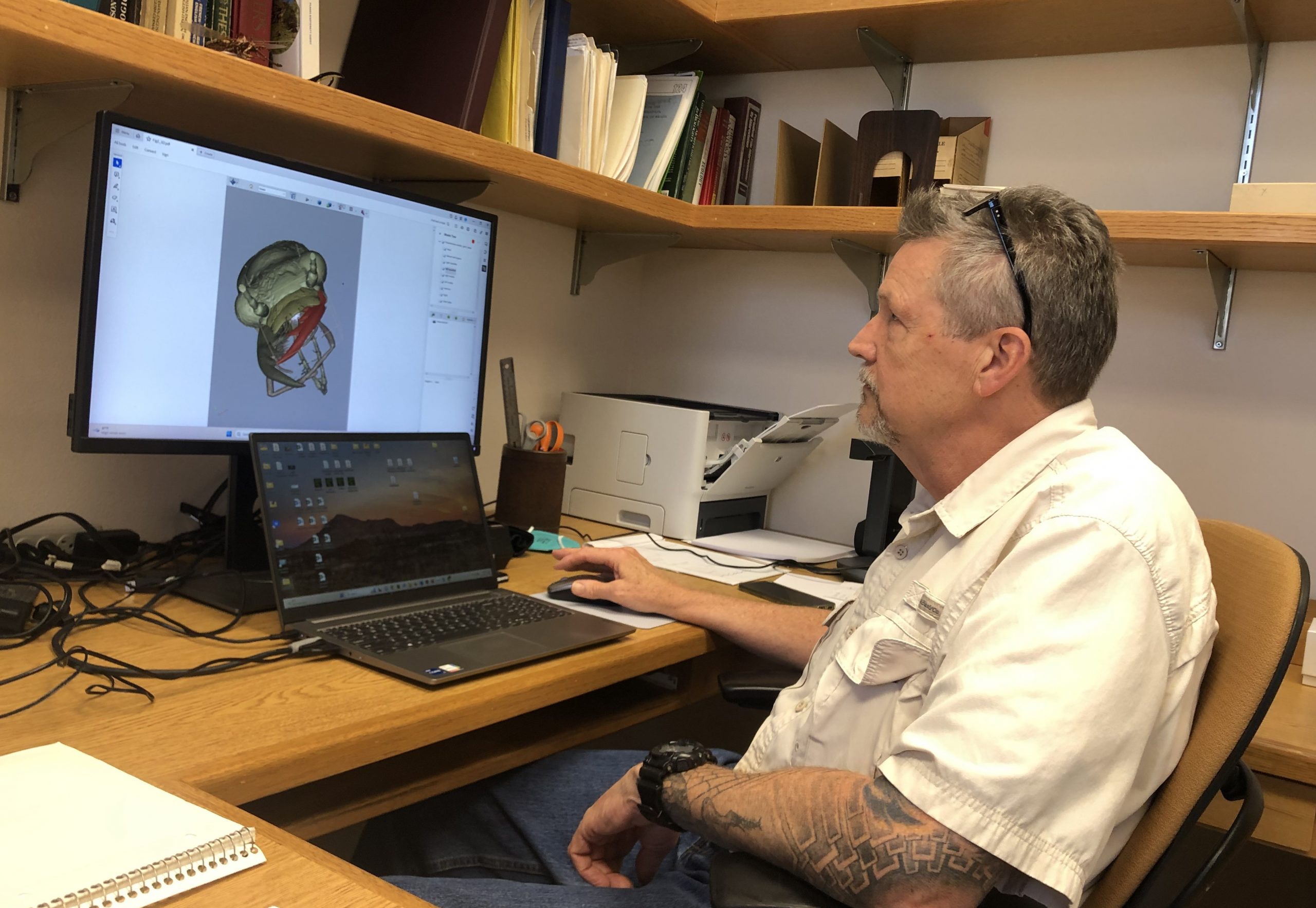
Professor Kip Will examining the model.
Story by:
Sloane Sim, Media Specialist
Essig Museum of Entomology